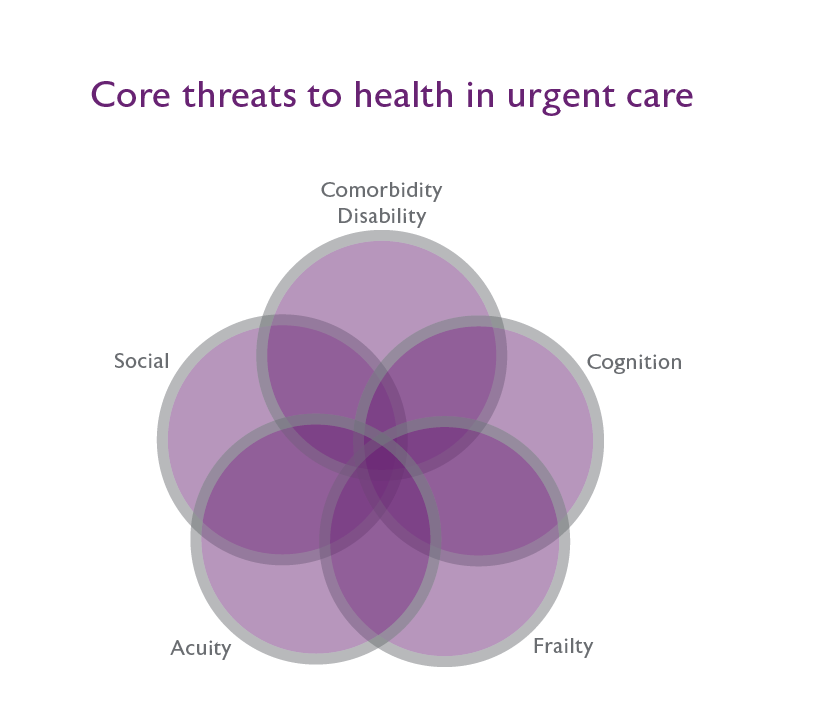
What is frailty?
Frailty is a condition characterised by loss of biological reserves, failure of physiological mechanisms and consequent increased risk of experiencing a range of adverse outcomes, including hospitalisation, longer length of inpatient stay, and delirium.5-7